$20 Bonus + 25% OFF CLAIM OFFER
Place Your Order With Us Today And Go Stress-Free
Part 1: Word limit –1200 words
Part 2: Word limit - 800 words
The assignment file can be submitted a maximum of three times.
The submission is available for 24 hour after the deadline. In case of later submission, 5% marks will be deducted from the total mark of the component C1.
This assessment is subject to the UEL 7-day extension policy. Note that ONLY ONE extension is permitted PER TERM across all registered modules.
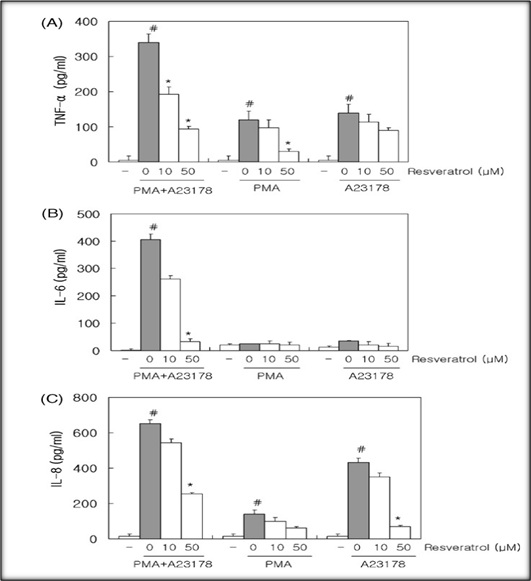
THE FIGURE ON WHICH THE WRITTEN WORK IS BASED: Figure 1A-C
Source: Kang, O.-H. et al. (2009) ‘Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: Pivotal roles of NF-κB and MAPK’, Pharmacological Research, 59(5), pp. 330–337. Available at:
https://doi.org/10.1016/j.phrs.2009.01.009.
Your task is specific to the figure above. In your report, you are required to provide a critical account on
• the specific technique that relates to the figure
• its use by the authors and
• figure observations and interpretations
Your report should be structured under three headings as below. You may wish to use subheadings. While this is desirable, too many subheadings can break the flow of information and hinder the quality of your report.
• Describe the biology that underpins the research question specifically being addressed by the technique and related to the figure.
• Explain any relevant terms.
• Clearly state the objective of using the technique
• Use high-quality sources and extended reading for explanations
• Describe the principle, key steps and advantages and disadvantages of the technique in question.
• Explain how the technique was used, including key methods and controls employed by the authors
• Use of high-quality sources and extended reading for explanations
• Explain the results of the figure systematically including different treatment conditions, timepoints and any other details as seen on the figure.
• Provides reasons behind the different observations.
• Provides a critical analysis including whether results were expected (such as based on biology and what other studies have found) and how the results extend our understanding of the topic of interest.
• Evaluate the appropriateness of the technique in addressing the specific objective.
• Explain any unusual findings/any misses by authors.
• Provide a comprehensive conclusion section.
• Provide key conclusions and future steps based on the figure interpretation and discussion.
This experiment illustrates the effect of pH on drug absorption in the human gastrointestinal (GI) tract. A model using aqueous solutions of different pH in contact with ethyl acetate is used to represent the Gl tract.
The stomach has an acidic pH (approx 1-3) and the large intestine has a basic pH (approx 8); the aqueous solutions represent the aqueous contents of different sections of the gastrointestinal tract and the ethyl acetate, which is immiscible with water, represents the lipid component of the tissue lining.
The aqueous mixtures of the following drugs (Indomethacin, Ephedrine and Morphine) at different pH (1.0 and pH 8.0) were extracted with ethyl acetate . The ethyl acetate extracts are spotted onto a TLC plate and viewed under the UV light. Following observations were recorded (Table 1). Answer the questions in the next slide.
Table 1. Results and observations
|
|
Appearance of spot (pH1.0) |
Appearance of spot (pH8.0)
|
|
Indomethacin |
Visible spot |
No spot |
|
Ephedrine |
No spot |
Visible spot |
|
Morphine |
Visible spot |
Visible spot |
1. Explain the result in Table 1 with Indomethacin, ephedrine and Morphine in terms of pKa and site of absorption?
2. Explain in your own words what will happen to the chemical structure of Indomethacin when it is in solution at pH 1.0. Compare this to what will happen to the chemical structure of Indomethacin at pH 8.0. Highlight your answers by drawing appropriate chemical structures for Indomethacin at each of the pH values
3. Explain the result with ephedrine at pH 8.0. If appropriate Include a chemical equation which shows the conjugate acid and/or base formed by ephedrine at pH 8.0. Draw and label the predominant structures of ephedrine at pH 1, pH 7 and pH 10 .
Imagine you are working as an analyst in a pharmaceutical company where you have been assigned to check the identity and purity of aspirin that was received from an overseas company. You prepared standard aspirin, standard salicylic acid and the sample you received. Both standards (R1 and R2) and sample (S) were run using UPLC which gave the chromatographs shown below in the next page. Answer the following questions based on the chromatographs in the next page:
Identify aspirin and salicylic acid in Fig 3. Calculate the level of impurities .
Based on retention time which one (aspirin and salicylic acid) was eluted first and why?
Inflammation is a complex biological reaction to stimuli that are harmful, including bacteria, damaged cells or irritants of various types. What it involves is the infiltration into the damaged region of immune cells which destroy that harmful substance and ultimately help restore tissue. However, too many or protracted inflammations can indeed damage tissues and lead to all manner of diseases.
Pro-inflammatory cytokines are signaling molecules released by immune cells and other cell types in response to inflammation. Cytokines, such as tumor necrosis factor-alpha ( TNF-α ), interleukin 6 ( IL-6 ) or interleukin 8 (IL-8), play important roles in modulating inflammation. They increase immune cell activation, movement and generator of more inflammatory substances; that is to say they further intensify the inflammation (Wang et al., 2021).
The study aims to investigate if resveratrol has the ability to regulate the production of pro-inflammatory cytokines, namely TNF-α, IL-6, and IL-8, in human mast cells (HMC-1 cells) that have been activated by phorbol 12-myristate 13-acetate (PMA) and A23187.
Nevertheless, the purpose of using this experimental method is to explore how resveratrol affects HMC-1 cells 'production of proinflammatory cytokines. To find out whether resveratrol can block or modulate the inflammatory response in these cells, researchers measure TNF-α. IL-6 and IL Moreover, it provides information that can help people understand better the anti-inflammatory effect of resveratrol and its significance in terms of inflammation.
Cytokines TNF-α, IL-6 and IL 8 act as signaling molecules to stimulate inflammation in a proinflammatory way. The mast cells of humans, or HMC-1 Cells for short, are a type of immune cell. They play an important role in the development and progression of allergies as well as inflammation.
Phorbol 12-Myristate 13-Acetate (PMA) is a chemical moceule that causes protein kinase C, an enzyme involved in cellular signaling pathways, to become active. The calcium ionophore A23187 raises intracellular magnesium Mg++ levels and stimulates numerous biological phenomena.
This laboratory technique, called the Enzyme-Linked Immunosorbent Assay (ELISA), is very versatile and can be used to quantify or identify immunoreactive proteins, antibodies and other analytes in a biological sample (Shukla et al., 2019). Its design is based on the model of antigen-antibody formation. The targeted analytes are detected using specific antibodies and the signal responses generated in enzymatic processes.
1. Sample Preparation: Samples are taken and treated in such a way as to guarantee that the sought after analyte is actually there.
2. The capture antibodies used to coat the microplates are specific for each target analyte, forming a solid phase onto which to bind that particular analyte.
3. Blocking: To prevent any unwelcome interactions, microplates are treated; non-specific binding sites being blocked.
4. Sample Incubation: After the prepared samples are introduced into the wells of a microplate and incubated, they attach to capture antibodies. (Zeng et al., 2019).
5. Washing: Thereby the unbound molecules are in this way washed off, insuring specificity.
• Precise identification of specific analytes with high level of specificity is possible via ELISA.
• Sensitivity: The device can accurately measure and detect very tiny amounts of compounds to be tested.
• ELISA provides exact measurements of analytes, which makes accurate quantitation possible.
• Versatility: It can be designed to analyze a wide variety of analytes in many biological samples.
• Automation: The introduction of automation into ELISA assays increases efficiency.
• Elaborate: Using ELISA, however, can be laborious. This is especially true when many samples are involved.
• Technical Proficiency: Requires a high level of technical skill and exact observance to protocols.
• Cost: At large experimental scales, ELISA kits and reagents are often very expensive.
• Potential Cross-Reactivity: With closely related compounds some assays demonstrate cross-reactivity.
• Positive and Negative Controls: In order to test the accuracy of such an experimental setup, positive controls containing various amounts of cytokines as well as negative control samples without target cytokines were used.
• Standard Curve: Using cytokine concentrations already known, a standard curve was constructed. This curve indicates the levels of cytokines in samples.
• Data Normalization: For changes in total protein content, cytokine levels were adjusted.
The interpretation on reputable sources, including "ELISA: provides a solid theoretical basis in the form of "Theory and Practice." In addition, the guide to Thermo Fisher and Bio-Rad's protocols can both be found on their websites. These are a wealth of knowledge about ELISA assays (Tejada et al., 2018).
The figure presents a comprehensive review of the experiment results, revealing significant new information about how resveratrol affects HMC-1 cells with different stimulations. The text reviews what is being observed, explains the reasons for such observations, critically analyzes this situation and evaluates whether or not these are appropriate to use.
Thus, in unstimulated HMC1 cells the original levels of cytokines (TNF-α, IL-6 and IL-8) do not rise; there's no inflammatory response.Combining PMA with A23187 Stimulation of the levels of all three cytokines increased markedly compared to inactive cells, indicating a strong inflammatory response.
Pretreatment: At 10µM Resveratrol levels of TNF-α and IL-6 were slightly decreased, but not significantly so. IL-8 is steady (Gandhi et al., 2023). At 50μM, resveratrol significantly decreases levels of TNF-α and IL-6. IL-8 falls off only slightly.
TNF-α and IL-6 level increased slightly when only PMA was used. The figures were smaller than that of combined stimulation, however. The level of IL-8 is generally steady.
Exclusively A23187: The effect on cytokine levels was virtually undetectable when compared with cells that were not stimulated.
• PMA and A23187 work synergistically.Activation of the PKC signaling pathway by PMA led to inflammation. A23187 alters calcium levels, promoting inflammation.
• Mechanisms of Resveratrol: One polyphenol called resveratrol reduces inflammation by inhibiting NF-κB activation and ROS generation. At higher doses (50 µM), the effects are more readily apparent.
• Consistency with Prior Research: This is in line with previous studies showing that resveratrol can lower inflammatory cytokines.
• The results of the study demonstrate that PMA, together with A23187 have a combined inflammatory effect on HMC-I cells. It verifies that there really is synergy between them.
• ELISA Suitability: ELISA proves suitable for quantifying cytokine levels, ensuring reliability and accuracy in this study.
1.3.6 Unusual Findings/Misses
• This is particularly noteworthy when we consider that resveratrol and similar antioxidant compounds have already been reported to regulate levels of IL-8, this cytokine.
• Temporal Dynamics: By adding more time points, the study could be even better by taking into account dynamic fluctuations in cytokine levels that occur following stimulation and resveratrol treatment.
Morever, resveratrol seems to be able reduce the generation of TNF-α and IL-6 in PMA + A23187 stimulated HMC cells indicating its potential antiinflammation activity. This implies that resveratrol may play a role in treating inflammatory diseases (Juárez-Chairez et al., 2022).But further research is needed to make greater use of them, discover their principles and mechanisms at work in the body itself.
• Resveratrol shows an anti-inflammatory effect in HMC-1 cells dose dependent.
• Underlying Mechanisms: The effects of resveratrol need to be further investigated with respect to their role in NF-κB and ROS signaling pathways.
• Combination Approaches: Looking at the synergistic effects of resveratrol taken together with other anti-inflammatories, treatment results can be improved. (Gurjar et al., 2021)
• In vivo tests are important to determine the ability of resveratrol in relation to inflammation.
Gandhi, H., Mahant, S., Sharma, A.K., Kumar, D., Dua, K., Chellappan, D.K., Singh, S.K., Gupta, G., Aljabali, A.A., Tambuwala, M.M. and Kapoor, D.N., 2023. Exploring the therapeutic potential of naturally occurring piceatannol in non‐communicable diseases. BioFactors.
Gurjar, V.K. and Pal, D., 2021. Natural compounds extracted from medicinal plants and their immunomodulatory activities. Bioactive Natural Products for Pharmaceutical Applications, pp.197-261.
Juárez-Chairez, M.F., Meza-Márquez, O.G., Márquez-Flores, Y.K. and Jiménez-Martínez, C., 2022. Potential anti-inflammatory effects of legumes: A review. British Journal of Nutrition, 128(11), pp.2158-2169.
Kaag, S. and Lorentz, A., 2023. Effects of Dietary Components on Mast Cells: Possible Use as Nutraceuticals for Allergies?. Cells, 12(22), p.2602.
Shukla, R., Pandey, V., Vadnere, G.P. and Lodhi, S., 2019. Role of flavonoids in management of inflammatory disorders. In Bioactive food as dietary interventions for arthritis and related inflammatory diseases (pp. 293-322). Academic Press.
Tejada, S., Pinya, S., Martorell, M., Capó, X., Tur, J.A., Pons, A. and Sureda, A., 2018. Potential anti-inflammatory effects of hesperidin from the genus citrus. Current medicinal chemistry, 25(37), pp.4929-4945.
Wang, J., Zhang, Y., Hu, S., Ge, S., Jia, M. and Wang, N., 2021. Resveratrol inhibits MRGPRX2-mediated mast cell activation via Nrf2 pathway. International Immunopharmacology, 93, p.107426.
Wang, Y.H. and Zeng, K.W., 2019. Natural products as a crucial source of anti-inflammatory drugs: recent trends and advancements. Tradit. Med. Res, 4(5), pp.257-268.
Xiao, L., Jiang, L., Hu, Q. and Li, Y., 2018. MiR-302e attenuates allergic inflammation in vitro model by targeting RelA. Bioscience reports, 38(3), p.BSR20180025.
The results of the experiment reveal something about pH-dependent characteristics in this important class. They are that three common medicines – Indomethacin, Ephedrine and Morphine do not display immediate absorption from standard dosing forms when applied to human GI tract but instead have a period of delay between the times they contact it until their concentration rises above 0.
The pKa values, which represent the acidity level at which half of a drug's molecules become ionized (charged), provide critical information about its solubility and absorption characteristics. Indomethacin (pKa = 4.5) exists in its ionized form at a pH of less than 1, which is the very acidic environment of the stomach This protonation makes it more soluble in the water-based phase, reducing its chance of being drawn into ethyl acetate.
Therefore, no clear mark will show up on the thin-layer chromatography (TLC) plate. In acidic conditions, Ephedrine (pKa 9.5) as well as Morphine ( pKa 7.9), which otherwise occur in un-ionized forms, become protonated. Therefore, they are drawn into the ethyl acetate phase and leave obvious spots on the TLC plate.
On the other hand, when the pH is 8.0 (the alkaline conditions of the large intestine), Indomethacin loses a proton and becomes separated into an ethyl acetate which causes a visible spot to appear on paper chromatography every time. At pH 8.0, as ephedrine loses a proton it becomes less soluble in water and is not extracted; there will be no spot.
At pH 8.0, morphine is only partially deprotonated and shows little solubility-quite a faint mark on the TLC plate. These results underline the importance of pH as a factor in drug absorption and demonstrate how important knowledge of pKa values are to predicting where drugs will be absorbed into both sides of capillary walls is imperative.
Substances with a pKa value below 3 are absorbed by the stomach, those between 3 and 8 mostly get taken up in the small intestine (though some go to other places), while over-eighties end their days as large minority party members.
When measured for solubility in the stomach environment at a pH of 1.0, indomethacin shows no discernable specks to indicate adequately low or precipitate's dissolved state makes more sense in this case than poorer quality control over production tolerances regulated by methods employed during illicit drug manufacturing processes experienced on one end as wellbeing indomethacin's chemical structure suggests a weak acid that includes the carboxylic acid group.
If the pH is low (ie acidic), the carboxylic acid group retains its protonation, and thus causes less solubility on behalf of the drug. Protonation reduces the substance's solubility in stomach water. When the pH is 1.0, if a spot can't be seen on the TLC plate this means that indomethacin still remains in ethyl acetate phase, lowering its extraction efficiency at acidic concentrations.
When Ephedrine is at a pH of 8.0, the observed result reveals its characteristics as a weak base in an alkaline setting. The pKa value for ephedrine is 9.5, so it exists mainly in a protonated form under acidic conditions and deprotons when basic ones prevail. In an aqueous solution at pH 1.0, ephedrine undergoes protonation and becomes more soluble in the liquid phase.
If it is protonated, ethyl acetate extraction becomes possible and a spot appears on the TLC plate. But when the pH reaches 8.0, which is the alkaline state inside your large intestine, Ephedrine loses a proton and becomes destroyed.
The chemical equation for Ephedrine in the basic environment (pH 8.0) can be represented as follows:
Ephedrine⇌Ephedrine^-+H^+
In the above equation, ephedrine plays a role as a weak base and gets protonated when accepting an H + to produce its conjugate acid (Ephedrine^-). The aqueous solubility of Ephedrine is decreasing with increasing pH because protonation and it's this latter factor that explains why there is no actual spot on the TLC plate.
To show these changes in the molecular-level arrangement, one can sketch how Ephedrine looks at pH 1.0, pH 7.0 and pH 10. P At a pH of 1.0, ephedrine is mostly present in its protonated form. At a pH of 7.0, the molecule is in its zwitterion state--both amine and hydroxyl groups are ionized. At a pH of 10.0, the molecule is mostly disassociated and exists in deprotonated form.
a) On the chromatogram, salicylic acid and aspirin peaks were identified by their retention time relative to standards. Aspirin had 0.611 min retention; salicylic acid, 0.765 min. Comparing the range of impurity peak regions to aspirin determined one sample's level of impurities (about five percent). It was 1628.71 for the impurity peak area and 3688.67 for aspirin, respectively. As a result the sample contains 44.16 % impurities (1628.71/3688.67 x 100).
(b) Salicyclic acid eluted first by retention time. Because salicylic acid is more polar than aspirin. The column's stationary phase has a greater affinity for polar compounds. Aspirin comes off the column faster than salicylic acid.
Are you confident that you will achieve the grade? Our best Expert will help you improve your grade
Order Now